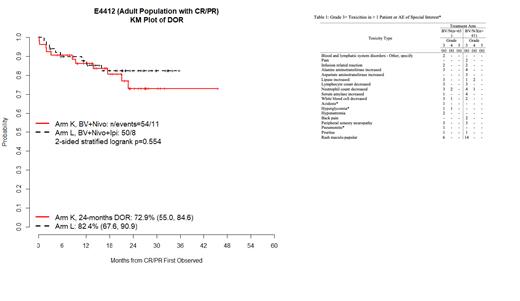
Background: Relapsed/refractory (R/R) Classic Hodgkin lymphoma (cHL) remains a significant clinical challenge. We hypothesized that using immune checkpoint blockade to activate the immune cells in the tumor microenvironment, and concurrently targeting tumor cells with the CD30 targeting antibody-drug conjugate brentuximab vedotin (BV) could overcome tumor resistance. E4412 is an intergroup Phase 1/2 ECOG-ACRIN sponsored study of the combinations of BV, nivolumab (N), and ipilimumab (I) in patients with R/R cHL. Here we present the efficacy and safety data on the full adult cohort of patients treated in Phase 2 randomized between BV/N and BV/N/I. Methods: Patients with confirmed R/R cHL were randomized between the doublet of BV/N with BV 1.8mg/kg and N 3mg/kg and BV/N/I with I 1mg/kg. BV and N were administered every 3 weeks, and I every 12 weeks for up to 1 year for BV and up to 2 years for N and I. Results: One hundred twenty-six patients were enrolled and treated; 8 patients (4 from each arm) were excluded from efficacy analysis based on ineligibility. Patients were not heavily pretreated, 65% had one prior line of therapy and an additional 27% had two prior lines, and 8% had more than 2 prior lines. The median age was 34; 51% of patients were male. Sixteen patients had prior BV: 10 (BV/N arm) and 6 (BV/N/I). Response: One hundred eighteen patients are evaluable for response: 61 treated with BV/N and 57 with BV/N/I. With BV/N 37 of 61 (60.7%) of patients had a complete response (CR) compared to 38 of 57 (66.7%) with BV/N/I for a difference of 6.7% (p-value 0.31). Additionally, 17 (28%) with BV/N and 12 (21%) of patients treated with BN/N/I had a partial response (PR) for an overall response rate (ORR) of 88% in both arms. In a planned analysis of patients with prior treatment with BV the CR rate was 25% in BV/N arm (2 of 8) and 60% in BV/N/I arm (3 of 5). The median follow-up for progression free survival (PFS), duration of response (DOR), and overall survival (OS) is 24.8 months and 33.7 months. The median PFS, duration of response and OS have not been reached for either arm with 24 months of follow-up ( Figure 1). In B/N 30 of 61 patients came off study to receive alternative treatments (primarily autologous stem cell transplant (SCT)) and in BV/N/I 23 of 57 patients did. Safety: Table 1 shows treatment-related Grade 3+ toxicity occurring in > 1 patient and adverse events (AE) of special interest. All 126 patients (118 eligible and 8 ineligible) are evaluable for safety. The rate of Grade 3+ treatment related toxicity is 43% (28/65) and 52% (32/61) for BV/N and BV/N/I, respectively. However, when rash is excluded the rate of treatment related toxicities is similar between the two arms 38.5% vs 39.3%. There was no grade 4 rash, and the rash was treatable in all cases with topical or oral steroids. There were no Grade 5 toxicities in either arm. Conclusion: In this randomized Phase 2 study comparing BV/N vs. BV/ N/I the difference in CR rate of 6.7% between the doublet and the triplet was not statistically significant. Additionally, the triplet had more grade 3 rash than the doublet, there was otherwise no significant difference in grade 3 or greater toxicity between the two arms. With a median follow-up of 24 months the PFS and DOR have not been reached for either arm, suggesting excellent disease control from both regimens. Differences in response for BV pre-treated patients between the two arms is intriguing especially as more patients are treated with BV in the frontline, but the sample size is too small to draw firm conclusions. Correlative studies are underway to elucidate a response vs. resistance signature, and biological understanding of differential benefit.
OffLabel Disclosure:
Diefenbach:Roche/Genentech: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Regeneron: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Genmab: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Beigene: Consultancy, Research Funding. Ansell:ADC Therapeutics: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research; Seagen Inc: Other: Contracted Research; Regeneron Pharmaceuticals Inc: Other: Contracted Research; Pfizer, Inc: Other: Contracted Research; Affirmed: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research. Steidl:Seattle Genetics, AbbVie, and Bayer: Consultancy; Bristol Myers Squibb, Epizyme and Trillium Therapeutics Inc.: Research Funding. Scott:Abbvie, AstraZeneca, Incyte: Consultancy; Janssen and Roche: Research Funding. Mehta-Shah:Secura Bio/Verastem: Consultancy, Research Funding; Genentech: Consultancy; Corvus Pharmaceuticals: Research Funding; C4 Therapeutics: Consultancy, Research Funding; Janssen: Consultancy; Karyopharm Therapeutics: Consultancy; Ono Pharmaceuticals: Consultancy; Genentech/Roche: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Bristol Myers-Squibb: Research Funding; Celgene: Research Funding; Innate Pharmaceuticals: Research Funding; AstraZeneca: Consultancy, Research Funding; Kyowa Hakko: Consultancy. Amengual:Astrazeneca: Consultancy; Epizyme: Honoraria; Incyte: Consultancy. Bartlett:ADC Therapeutics, Autolus, BMS/Celgene, Forty Seven, Gilead/Kite Pharma, Janssen, Merck, Millennium, Pharmacyclics, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Research Funding; ADC Therapeutics, Foresight Diagnostics, Kite, F. Hoffmann-La Roche Ltd / Genentech, Inc., Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Washington University School of Medicine: Current Employment. Advani:Cyteir: Research Funding; Regeneron: Research Funding; Epizyme: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Beigene: Membership on an entity's Board of Directors or advisory committees; ADCT: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Research Funding; Seagen: Research Funding. Ibrahimi:Ipsen Bio, BMS, Sobi Inc: Consultancy. Kahl:ADCT: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Genmab: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Lilly: Consultancy, Honoraria.
Use of ipilimumab in combination with brentuximab and nivolumab in R/R HL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal